Will FDA-Approved Microchips in Humans Cause Cancer?
by www.SixWise.com
About 2,000 people around the world have been implanted with
radio
frequency identification (RFID) devices manufactured by
VeriChip Corporation, and now studies have revealed that the
microchips may be linked to cancer.
|
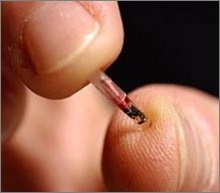
New revelations that implantable microchips may cause
cancer in animals raises safety concerns for the 2,000
people who have already received the chips.
|
Similar tiny microchips, which are about the size of two
grains of rice, are now commonly implanted into pets to help
owners locate them if they get lost. A simple scan of the
chip by a veterinarian or animal shelter reveals its encoded
information, such as the owner's name and contact information.
In a similar vein, the microchips are now being touted as
a medical innovation that could transmit important medical
information in people with Alzheimer's disease and other conditions
that make identification difficult. A scan of the patient's
arm would reveal the person's entire medical history almost
instantly.
So promising was the potential for these microchips to save
lives that the U.S. Food and Drug Administration (FDA) approved
the first RFID chips that can be implanted into humans in
October 2004. Then, in 2005, the FDA praised the microchip
as "one of 2005's top innovative technologies."
Already the chips have branched off into non-medical uses
-- staff members of the Mexican Attorney General's office
have been implanted with the VeriChip to control access to
a data room, for instance, and two nightclubs overseas use
an implantable VeriChip to identify VIP customers and allow
them to buy drinks.
However, VeriChip is working on expanding the chip's medical
market to a target audience of 45 million Americans, particularly
those with Alzheimer's disease, heart conditions and diabetes.
Already, they've invested millions in assembling a national
network of hospitals that are equipped to scan patients with
microchips.
How Does the Human Microchip Work?
Similar to the microchips in pets, the chip is implanted
into a person's upper arm using a syringe. When the chip is
scanned, it reveals a unique code that allows health care
officials to access the person's medical records via an online
database.
The database is maintained by VeriChip, and requires an annual
fee.
Animal Studies Reveal Cancer Risk
When the FDA approved the implantable RFID chips for people,
they revealed the following potential complications:
-
Adverse tissue reaction
-
Migration of the implanted transponder
-
Failure of implanted transponder
-
Electrical hazards
-
Possible incompatibility with magnetic resonance imaging
(MRI)
They did not, however, mention any of the studies that The
Associated Press (AP) revealed, which found a potential link
between the microchips and cancer.
After a review of studies published in veterinary and toxicology
journals between 1996 and 2006, the AP found that the chips
"induced malignant tumors in some lab mice and rats."
|

VeriChip, which is about the size of two grains of
rice, is implanted in a patient's upper arm using a
syringe.
|
"The transponders were the cause of the tumors,"
Keith Johnson, a retired toxicologic pathologist, told the
AP in a phone interview about one 1996 study that he led.
The AP also detailed the following studies:
-
A 1998 study of 177 implanted mice that found a cancer
rate of just over 10 percent
-
A 2006 study that detected tumors in 4.1 percent of 1,260
microchipped mice
-
A 1997 study that found cancers in 1 percent of 4,279
microchipped mice
The AP had these studies reviewed by leading cancer specialists,
who agreed that although animal studies don't necessarily
mean the same thing will happen to humans, the results were
troubling.
"There's no way in the world, having read this information,
that I would have one of those chips implanted in my skin,
or in one of my family members," said Dr. Robert Benezra,
head of the Cancer Biology Genetics Program at the Memorial
Sloan-Kettering Cancer Center in New York in an AP article.
The FDA said that they did take into account animal studies
when reviewing the human microchip for approval, but they
did not see any scientific studies linking the chips to cancer
in dogs or cats. Lab rodents, the FDA says, are more prone
than humans and other animals to develop tumors from injections.
"At this time there appears to be no credible cause
for concern," said Karen Riley, a spokeswoman for the
FDA in a New York Times article.
As for VeriChip, the company said they were not aware
of the studies.
"We recognize that we have a corporate responsibility
to review these studies, to look at other studies, to do new
studies if necessary, and do what is appropriate after reviewing
all the information in all regards, and we intend to do this,"
said Scott Silverman, the chairman and CEO of VeriChip.
The cancer specialists that reviewed the AP studies said
that more studies are needed, on dogs and non-human primates,
before the microchips should be assumed safe for human use.
"I mean, these are bad diseases. They are life-threatening,"
said Dr. Benezra. "And given the preliminary animal data,
it looks to me that there's definitely cause for concern."
They also agreed that the cancer link found in the animal
studies should be disclosed to anyone considering the chip.
The potential cancer link has also prompted concern from
pet owners wondering if the microchips are safe for their
animals. Several veterinarians say that the risk of getting
lost is a bigger concern than the potential cancer risk.
"The risk of getting lost and ending up at the pound
and maybe getting put to sleep is a bigger risk in the big
picture," said Dr. Nicholas Dodman of the Tufts Cumming
School of Veterinary Medicine in an ABC News article.
Recommended Reading
RFIDs:
The Pros and Cons Every Consumer Needs to Know About Radio
Frequency Identification Tags
Proposed
National Database Sparks Privacy Controversy
Sources
Bradenton
Herald September 9, 2007
ABC
News September 10, 2007
The
New York Times September 11, 2007